[rev_slider alias=”servicos”]
[/rev_slider]
Doing Business in Brazil
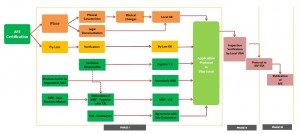
To make business in the Brazilian market is mandatory the City Hall VISA & Federal License from ANVISA – “AFE”.
See in the flowchart bellow the sequence to be followed.
WE CAN PROVIDE ALL THAT YOUR COMPANY WILL NEED TO DO BUSINESS IN BRAZIL AND GET ALL THE APLLYIED LICENSES.
-
ANVISA LICENSE – AFE
ANVISA License – AFE
is necessary for doing business involving the above products and the following activities:
– Industry – Manufacturing facilities,
– Importation and Distribution
– Importing for/by third parties,
– Distribution Centers,
– Carriers,
– Storage.
-
BEST PRACTICES MANUAL – MBP
BEST PRACTICES MANUAL – MBP
In order to submit the Company Legal Documentation is necessary to prepare and implement the Best Practices Manual – MBP – for the chosen activity:
- Prepare a detailed plan for implementation
- Preparing your team and relevant documentation;
- Following up the implementation plan and assist your team
- Lead an Internal simulated Auditing;
- Analyze all the documentation needed;
- Submit the Legal Documentation to VISA Authority (City Hall and/or Federal)
We can also provide the Certification of Best Practices when it is necessary.
-
PRODUCT REGISTRATION
PRODUCT REGISTRATION
In Brazil, the National Sanitary Surveillance Agency (ANVISA) must approve the vast majority of products related to “health products” before marketing.
Interventus can analyze, develop, assist, review and submit to ANVISA the products dossiers that the company want to market.
Interventus will follow up the all the processes until its publication in the Official Gazette (D.O.U.).
The Product process will follow the ANVISA’s Rules (RDC’s) and according to its classification:
- Registration;
- Notification;